Overview
Nose2Knee represents a significant advancement in cartilage tissue engineering, leveraging the unique properties of nasal chondrocytes to repair knee cartilage defects. Traditional approaches often use cells from the knee itself, but these methods can be limited by donor site morbidity and variable cell quality. Nose2Knee overcomes these limitations by sourcing chondrocytes from the nasal septum, which exhibit superior regenerative capacity and resilience.
Scientific Rationale
Nasal chondrocytes possess remarkable proliferative and chondrogenic abilities, outperforming articular chondrocytes in both laboratory and clinical settings. They are less affected by patient age and can thrive in the inflammatory environment of injured or osteoarthritic joints. This makes them ideal for engineering durable cartilage grafts.
Procedure
Biopsy: A small nasal cartilage sample (typically 6 mm) is harvested under local anesthesia.
Cell Expansion and Tissue Engineering: The chondrocytes are isolated, expanded in vitro, and seeded onto a collagen scaffold. Over about two weeks, they form a mature cartilage tissue (N-TEC) ready for implantation.
Implantation: The engineered graft is shaped to fit the knee defect and implanted via arthrotomy. The mature tissue provides immediate mechanical stability and protects cells from joint inflammation.
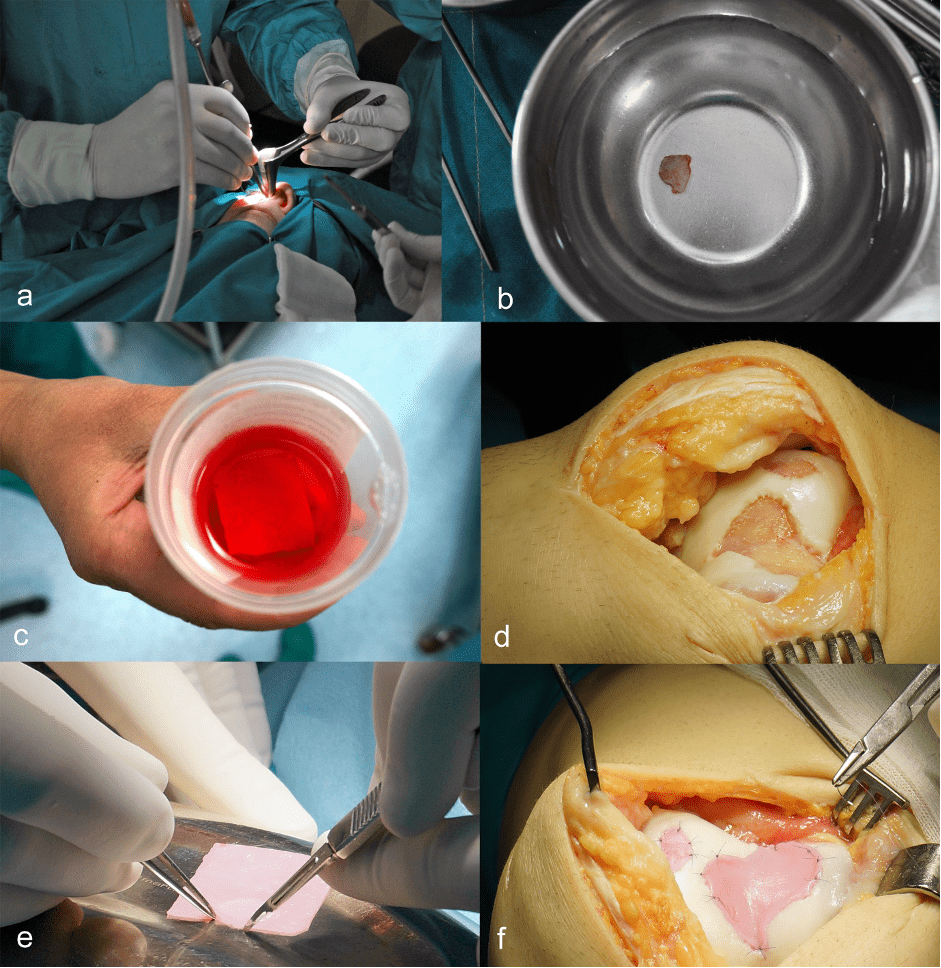
Figure 1. a) Biopsy of nasal cartilage under local anesthesia; b) A cartilage fragment of 7×7 mm is transported to a GMP laboratory; c) Cultivated autologous cartilage is delivered in a special medium; d) Debridement of all unstable parts of the lesion and preparation of the graft bed; e) Shaping of the graft according to the defects; f) Fixation of the graft with trans-cartilaginous sutures using monofilament, resorbable thread size 5-0 (adapted from Ivković A. Mogućnosti liječenja oštećenja zglobne hrskavice u sportaša. In: Sportska medicina, ur. Pećina M, Medicinska naklada, Zagreb, 2025.)
Clinical Evidence
Phase I/II Trials: Early clinical trials have demonstrated the safety and efficacy of Nose2Knee. Patients with full-thickness cartilage lesions showed significant improvements in pain, mobility, and quality of life, with no serious adverse events.
Comparative Outcomes: Mature nasal cartilage grafts yielded better clinical and MRI outcomes than less mature or traditional cell-based grafts, particularly in larger or previously treated defects.
Inflammatory Resistance: Nasal chondrocyte-derived tissues maintain their properties even in pro-inflammatory environments, suggesting potential for broader use in osteoarthritis.
Future Directions
Ongoing studies are expanding the Nose2Knee approach—originally developed for treating localized cartilage defects—to more complex cases, including osteoarthritis and patellofemoral cartilage defects, with the ultimate aim of offering a viable alternative to joint replacement surgery.
A major driver of this research is the ENCANTO project (ENgineered CArtilage from Nose for the Treatment of Osteoarthritis)( https://encanto.health/), a large-scale, international, multicenter phase II clinical trial funded by the European Union under the Horizon Europe program. ENCANTO is coordinated by Prof. Gianluca Vadalà at the Campus Bio-Medico University of Rome and is being conducted in 11 leading clinical centers across Europe. The project is specifically focused on patellofemoral osteoarthritis (PFOA), a challenging form of knee arthritis, and aims to clinically validate the use of nasal chondrocyte tissue-engineered cartilage (N-TEC) as a regenerative treatment. ENCANTO is expected to bring the first disease-modifying therapy for cartilage degeneration to the market, with the potential to restore joint function, reduce pain, and improve quality of life for patients—potentially delaying or even avoiding the need for joint replacement.
In parallel, the PFOA II study—funded by the Swiss National Science Foundation (SNSF)—is investigating the efficacy of N-TEC compared to standard therapies such as platelet-rich plasma (PRP) injection (https://dkf.unibas.ch/en/aktuell/pfoa-2-management/). This randomized, controlled, multicenter phase II trial includes 75 participants across 9 centers in Switzerland, Germany, and Croatia. The study design emphasizes rigorous follow-up and comprehensive clinical evaluation to thoroughly assess the long-term benefits of nasal cartilage-based therapy for patellofemoral osteoarthritis.
If these trials are successful, the Nose2Knee approach could revolutionize the management of cartilage injuries and degenerative joint diseases, offering a regenerative alternative to invasive joint replacement and significantly improving outcomes for patients worldwide.
Conclusion
Nose2Knee is a promising, evidence-based tissue engineering strategy that harnesses the regenerative power of nasal chondrocytes to restore knee cartilage. It offers improved outcomes, reduced donor site harm, and potential applicability in both traumatic and degenerative cartilage conditions.
References
- Acevedo Rua, L., Mumme, M., Manferdini, C., Darwiche, S., Khalil, A., Hilpert, M., Buchner, D. A., Lisignoli, G., Occhetta, P., von Rechenberg, B., Haug, M., Schaefer, D. J., Jakob, M., Caplan, A., Martin, I., Barbero, A., & Pelttari, K. (2021). Engineered nasal cartilage for the repair of osteoarthritic knee cartilage defects. Science translational medicine, 13(609), eaaz4499. https://doi.org/10.1126/scitranslmed.aaz4499
- Fulco, I., Miot, S., Haug, M. D., Barbero, A., Wixmerten, A., Feliciano, S., Wolf, F., Jundt, G., Marsano, A., Farhadi, J., Heberer, M., Jakob, M., Schaefer, D. J., & Martin, I. (2014). Engineered autologous cartilage tissue for nasal reconstruction after tumor resection: an observational first-in-human trial. Lancet (London, England), 384(9940), 337–346. https://doi.org/10.1016/S0140-6736(14)60544-4
- Mumme, M., Barbero, A., Miot, S., Wixmerten, A., Feliciano, S., Wolf, F., Asnaghi, A. M., Baumhoer, D., Bieri, O., Kretzschmar, M., Pagenstert, G., Haug, M., Schaefer, D. J., Martin, I., & Jakob, M. (2016). Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet (London, England), 388(10055), 1985–1994. https://doi.org/10.1016/S0140-6736(16)31658-0
- Mumme, M., Steinitz, A., Nuss, K. M., Klein, K., Feliciano, S., Kronen, P., Jakob, M., von Rechenberg, B., Martin, I., Barbero, A., & Pelttari, K. (2016). Regenerative Potential of Tissue-Engineered Nasal Chondrocytes in Goat Articular Cartilage Defects. Tissue engineering. Part A, 22(21-22), 1286–1295. https://doi.org/10.1089/ten.TEA.2016.0159
- Mumme M, Wixmerten A, Ivkovic A, Peretti GM, Yilmaz T, Reppenhagen S, Pullig O, Miot S, Izadpanah K, Jakob M, Mangiavini L, Sosio C, Vuletić F, Bieri O, Biguzzi S, Gahl B, Lehoczky G, Vukojevic R, Häusner S, Gryadunova A, Haug M, Barbero A, Martin I. Clinical relevance of engineered cartilage maturation in a randomized multicenter trial for articular cartilage repair. Sci Transl Med. 2025 Mar 5;17(788):eads0848. doi: 10.1126/scitranslmed.ads0848. Epub 2025 Mar 5. PMID: 40043142.
- Pelttari, K., Pippenger, B., Mumme, M., Feliciano, S., Scotti, C., Mainil-Varlet, P., Procino, A., von Rechenberg, B., Schwamborn, T., Jakob, M., Cillo, C., Barbero, A., & Martin, I. (2014). Adult human neural crest-derived cells for articular cartilage repair. Science translational medicine, 6(251), 251ra119. https://doi.org/10.1126/scitranslmed.3009688
- Šećerović A, Pušić M, Kostešić P, Vučković M, Vukojević R, Škokić S, Sasi B, Vukasović Barišić A, Hudetz D, Vnuk D, Matičić D, Urlić I, Mumme M, Martin I, Ivković A. Nasal Chondrocyte-Based Engineered Grafts for the Repair of Articular Cartilage “Kissing” Lesions: A Pilot Large-Animal Study. Am J Sports Med. 2021 Jul;49(8):2187-2198. doi: 10.1177/03635465211014190. Epub 2021 May 28. PMID: 34048271.
- Wixmerten, A., Miot, S., Bittorf, P., Wolf, F., Feliciano, S., Hackenberg, S., Häusner, S., Krenger, W., Haug, M., Martin, I., Pullig, O., & Barbero, A. (2023). Good Manufacturing Practice-compliant change of raw material in the manufacturing process of a clinically used advanced therapy medicinal product-a comparability study. Cytotherapy, 25(5), 548–558. https://doi.org/10.1016/j.jcyt.2023.01.003



